
Importing equipment and procedures
Arguably, equipment procedures (calibration, standardization, check, maintenance) are the most time-consuming and important part of any quality management system. Within our laboratory, we have 150+ pieces of equipment that will require about 300 procedures to be completed annually.
Initially, when adding equipment inventory with their procedures, and adding them one at a time, the process can also be extremely time-consuming. In this post, we are going to outline a recent upgrade that has been added that includes the ability to add procedures to each individual piece of equipment.
This new feature dovetails nicely with our previous user question, Q: We love the fact that we were able to import our equipment inventory, what about importing or adding equipment procedures (EP), how does that work?
This possible function has been asked about quite frequently of late, especially by laboratories with large inventories.
So, let’s take a look at the ease of importing your inventory while including equipment procedures at the same time:
First, log in to the application and click the Inventory tab where you will find a link on the upper right named “Import Inventory.” Click on “Import Inventory” and a dialog box will appear. Within the dialog box, you will then find the “Download Template” to add your equipment inventory.
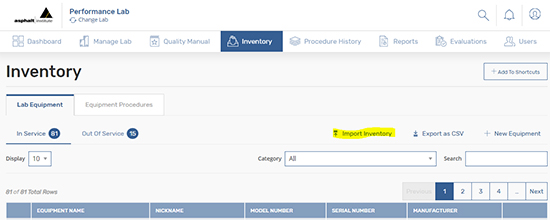
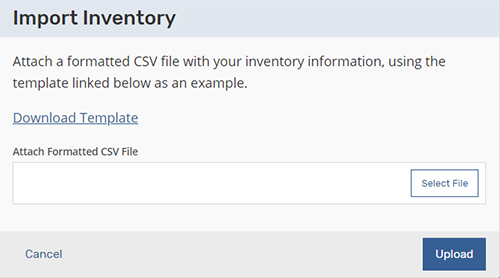
A few things to note on the download template
Equipment-related fields:
• Columns A-P
• The header will replicate the fields within the equipment details in the application.
• Required fields will be highlighted with an asterisk*
• Missing fields that are required will terminate the import.
• If there’s a required field and the user doesn’t have content, mark NA.
Procedure fields:
• Starting with column Q:
– Procedure 1, upload ID; this can be found under Manage Lab/equipment procedures widget/upload ID left column.
– Procedure 1, next procedure date; this can be determined based on the last completed procedure and frequency of the procedure.
– You can add up to 4 procedures.
• The column starting with Q relating to equipment procedures will not stop the import, if there is an incorrect procedure upload ID, at the completion of the import there will be a message that will note import results:

Another big topic is ‘creating and managing’ Calibration, Standardization, Check and Maintenance Procedures. So next month’s blog we will continue this discussion:
User Question
Q: What other services does R18LabQMS provide regarding training and ongoing assistance with our Quality Manual? We always seem to be shorthanded with keeping up, R18LabQMS has been a big help.
A: Great question. I’m always asked, “How long does it take for complete implementation?” The answer is always, it depends on the size of your lab (equipment inventory), and the amount of effort you want to dedicate to the process. That said, the application is intuitive, especially with the setup process. Now to answer your question specifically; we offer virtual training in one-hour segments at no additional cost, and on-site training in your lab for one day. There is a fee associated with this, so please inquire. As far as ongoing assistance, we are here to help wherever possible.
Bonus content for our R18LabQMS users
Passwords should be 8-70 characters with upper- and lower-case letters and numbers.
Resources
Once you work through the setup process, the following links can be found in the lower right-hand corner of the application.
Training – onboarding process
Getting started with R18LabQMS and what we provide regarding training, both virtual and onsite. Before we dive into options, getting started happens within the setup process, it’s intuitive and follows the sections that require procedures within the management and technical sections. Each section number within the setup process corresponds to AASHTO R18. You can either enter your procedure Itself for each section or upload a file with the details of the procedure. I always tell labs during a demo it’s much like a ‘Turbo Tax’ experience, it walks you through each step.
Once you have completed the setup process, or wish to move on to the dashboard view, just click save progress and exit setup in the upper right-hand corner. It’s straightforward and intuitive.
Upon exiting the setup process, there will be a dialog box that pops up asking you if you would like to add additional ASTM quality standards, and their sub-standards, i.e. D3666 (aggregate).
Additional Training needed to jump-start the onboarding process:
Virtual Training
We target a specific area for training and limit the time to one hour. An example would be adding Equipment Procedures to your Equipment. There is no charge for virtual training limited to 1 hour.
Onsite Training
We spend one day in your laboratory to review all aspects of your Quality Manual and take a deep dive into R18labQMS implementation. This training is fee-based, please inquire.
Upcoming meetings we will soon be attending and sponsoring:
ASTM sponsor ‘Working Lunches’
Denver, CO, June 6-8, 2023
AAPT sponsor
San Diego, CA, September 12-15, 2023
AASHTO re:source Q&A Podcast with Brian Johnson & Kimberly Swanson
S2 E18: Moving to a Digital Quality Management System
Gary Irvine, from the Asphalt Institute, joins us to discuss the benefits of moving from a paper QMS to a digital system.
Please check out our most recent promotional R18LabQMS video.
For all questions regarding your quality management system, whether your lab is accredited or not, please contact us and learn how R18LabQMS can add value to your laboratory.
One last favor to ask, if there is a specific topic of discussion you would like to see in upcoming blog posts, please send us a note.
Gary Irvine
R18LabQMS Program Manager